Showing posts with label cocaine addiction. Show all posts
Showing posts with label cocaine addiction. Show all posts
Saturday, October 31, 2015
Freud and his Drug Demons
Cocaine addiction and psychoanalysis.
That Sigmund Freud was a cocaine abuser for some portion of his professional life is by now well known. Reading An Anatomy of Addiction by Howard Markel, M.D., which chronicles the careers of Freud and another famed cocaine abuser, Johns Hopkins surgeon William Halsted, I was struck by the many ways in which even the father of modern psychotherapy could not see the delusions, evasions and outright lies that were the byproducts of his very own disease of the body and mind: drug addiction. Markel makes the case that in several important ways Freud’s cocaine addiction was hopelessly entangled with, and partially responsible for, his theorizing about the workings of the mind.
In 1884, Freud published his book, On Coca, a treatise on the wonders of cocaine. To his fiance, he wrote: “I have other hopes and intentions about [cocaine]. I take very small doses of it regularly against depression and against indigestion and with the most brilliant of success.” The book, a comprehensive review of cocaine’s effects, had an “n of 1”: “I have carried out experiments and studied, in myself and others, the effect of coca on the healthy human body.”
One of the defects of On Coca was its assertion that the drug was an effective antidote to serious morphine and alcohol abuse. Most astonishingly, however, Freud “skimmed over cocaine’s most important clinical use as a local anesthetic.” That discovery was later championed by ophthalmologist Carl Koller, whom Freud never forgave, even though the mistake was Freud’s alone. It seems reasonable to suggest that a moody doctor, who happened to be treating a close friend for morphine addiction at the time, might tend to focus on cocaine’s use against depression and drug abuse. And two years later, Freud vigorously fought back against an influential American doctor’s unambiguous assertion that cocaine was addictive. The U.S. physician had written that a “doctor self-prescribing cocaine was the equivalent of the lawyer representing himself in court: each had a fool for a patient or client.”
Markel notes that it is “also telling that he does not reveal to [his fiancé] the precise amount of cocaine he was ingesting. In fact, throughout his notes during this period, Freud minimizes the amount and frequency of his cocaine dosage, using such terms as ‘a little cocaine’ or a ‘bit of cocaine,’ a tactic many substance abusers employ to avoid the disapproval or intervention of others.”
Writing in his capacity as a physician, Markel states:
In light of the physical symptoms Freud suffered during this period, in my medical opinion, there is ample evidence that he was abusing significant amounts of cocaine during the early 1890s and that he was using it in a dependent, if not outright addictive fashion. In fact, cocaine likely had a negative effect on virtually every aspect of Sigmund’s personal relationships, behavior, and health. We can make such a declarative statement because his letters to Wilhelm Fleiss tells us precisely so…. Sigmund explained that he was suffering from a Fliessian syndrome of ‘crossed reflexes’ of the nose, brain, and genitals that had led to severe migraine headaches. The excruciating pain, not surprisingly, could only be interrupted by the multiple doses of cocaine prescribed by Dr. Fliess.
It was not pretty: “From a diagnostic standpoint, Sigmund’s nasal stuffiness is intriguing… Sigmund’s need for cauterization—the placement of a hot knife against swollen, blocked nasal tissue to, literally, burn open a passage for air—in concert with his disinclination to write suggests serious cocaine abuse.” And also telling is Freud’s habit of smoking 20 or more cigars each day.
By 1894, Markel writes, “the cardiac symptoms associated with cocaine use and the severe depression and headaches after its use—similar to what Sigmund was experiencing—were finally being reported in the medical journals of the day.” And, much like an alcoholic explaining away his chronic stomach troubles, “Freud continued to search for alternative explanations for his chest pain rather than seriously contemplate cocaine’s potential role in the matter.”
For readers in need of socioenvironmental triggers for addiction, Freud had a ready supply: “risk taking, resentments, loneliness, alienation, emotional pain, traumatic family experiences, phobias, neuroses, depression, denials and secretiveness about his sexuality, a possible sexual relationship with his sister-in-law, a brief flirtation with excessive drinking, and his self-documented cocaine abuse, to name some of his demons.”
About 1896, Freud stopped discussing his use of cocaine, and more or less dropped the subject altogether. Later in life, he speculated on whether his love of cigars (which eventually killed him) had helped keep him away from the task of working out his own psychological problems. “One wonders,” writes Markel, “whether his compulsive cocaine abuse from 1884 to 1896 was one of those unexplored problems.”
From 1896 to 1900, presumably cocaine-free years, Freud suffered from “depression, cocaine urges, occasional binge drinking, sexual affairs, caustic behaviors, and emotional absence.” To Markel, this adds up to the classic portrait of a “dry drunk,” AA’s description of someone who has given up drinking and drugs, and is miserable about it, and is making everyone around them miserable as well.
Markel points to the theory promulgated by historian Peter Swales to the effect that Freud’s entire concept of the libido “is merely a mask and a symbol for cocaine; the drug, or rather its invisible ghost, haunts the whole of Freud’s writing to the very end.”
Labels:
addiction,
coca,
cocaine addiction,
Freud and cocaine,
Halsted
Friday, January 23, 2015
The Losing Battle For Perpetual Reward
Or why you can't stay high forever.
The amphetamine high, like the cocaine high, is a marvel of biochemical efficiency. Stimulants work primarily by blocking the reuptake of dopamine molecules in the synaptic gap between nerve cells. Dopamine remains stalled in the gap, stimulating the receptors, resulting in higher dopamine concentrations and greater sensitivity to dopamine in general. Since dopamine is involved in moods and activities such as pleasure, alertness and movement, the primary results of using cocaine or speed—euphoria, a sense of well being, physical alertness, and increased energy—are easily understood. Even a layperson can tell when lab rats have been on a meth binge. The rapid movements, sniffing, and sudden rearing at minor stimuli are not that much different in principle from the outward signs of meth intoxication among higher primates.
Chemically, cocaine and amphetamine are very different compounds. Psychoactively, however, they are very much alike. Of all the addictive drugs, smoked cocaine and speed have the most direct and most devastatingly euphoric effect on the dopamine systems of the brain. Cocaine and amphetamine produce rapid classical conditioning in addicts, demonstrated by the intense cravings touched off by such stimuli as the sight of a building where the user used to buy or sell. Environmental impacts of this nature can produce marked blood flow increases to key limbic structures in abstinent addicts.
In clinical settings, cocaine users have a hard time distinguishing between equal doses of cocaine and Dexedrine, administered intravenously. As we know, it is the shape of the molecule that counts. The amphetamines are shaped like dopamine and norepinephrine, two of the three reward chemicals. Speed, then, is well suited to the task of artificially stimulating the limbic reward pathway. Molecules of amphetamine displace dopamine and norepinephrine in the storage vesicles, squeezing those two neurotransmitters into the synaptic gap and keeping them there. By mechanisms less well identified, cocaine accomplishes the same feat. Both drugs also interfere with the return of dopamine, norepinephrine, and serotonin molecules to their storage sacs, a procedure known as reuptake blocking. Cocaine works its effects primarily by blocking the reuptake of dopamine.
Amphetamine was once one of the most widely prescribed drugs in the pharmacological cornucopia. It exists in large part now as a recreational drug of choice, abuse, and addiction. The same is true of cocaine. It was replaced as a dental anesthetic long ago, in favor of non-addictive variants like Novocain. The same tragic list of statistical side effects that apply to abusers of alcohol, heroin and nicotine also apply to stimulant abusers: Increased risk of car accidents, homicides, heart attack, and strokes.
In the late 1990s, scientists at Johns Hopkins and NIDA showed that opiate receptors play a role in cocaine addiction as well. PET scans demonstrated that cocaine addicts showed increased binding activity at mu opiate receptors sites in the brain during active cocaine addiction. Take away the cocaine, and the brain must cope with too many empty dopamine and endorphin receptors. It is also easy to understand the typical symptoms of cocaine and amphetamine withdrawal: lethargy, depression, anger, and a heightened perception of pain. Both the cocaine high and the amphetamine high are easily augmented with cigarettes or heroin. These combinations result in “nucleus accumbens dopamine overflow,” a state of neurochemical super saturation similar to the results obtained with the notorious “speedball”—heroin plus cocaine.
With the arrival of smokable forms of cocaine and amphetamine, the race to pin down the biology of stimulation became even more urgent. Stimulants in smokable form—crack and ice—are even more rapidly addictive for addiction-prone users. “The reason has to do with the hydraulics of the blood supply,” a researcher at the University of Minnesota explained to me. “High concentrations are achieved with each inhalation, and sent right upstairs to the brain—but not all of the brain simultaneously. The target of the flow of blood is the limbic system, whereas the remainder of the brain is exposed to much milder concentrations.”
This extraordinarily concentrated jolt to the reward center is the reason why smokable cocaine and speed are able to pack such a wallop. The entire range of stimulative effects hits the ventral tegmental area and associated reward regions of the brain in seconds, and the focused nature of the impact yields an astonishingly pleasurable high.
But the long-term result is exactly the opposite. It may sound dour and religious, but the scientific fact of the matter is that continuous chemical pleasure extracts its fee in the end: The body’s natural stock of these neurotransmitters starts to fall as the brain, striving to compensate for the artificial flooding of the reward center, orders a general cutback in production. At the same time, the receptors for these neurotransmitters become excessively sensitive due to the frequent, often unremitting nature of the stimulation.
“It’s clear that cocaine causes depletion of dopamine, norepinephrine, serotonin—it is a general neurotransmitter depleter,” said my research source. “That may account for many of the effects we see after someone has stopped using cocaine. They’re tired, they’re lethargic, they sleep; they may be depressed, moody, and so on.” Continued abuse of stimulant drugs only makes the problem worse. One reason why cocaine and amphetamine addicts will continue to use, even in the face of rapidly diminishing returns, is simply to avoid the crushing onset of withdrawal. Even though the drugs may no longer be working as well as they once did, the alternative—the psychological cost of withdrawal—is even worse. In the jargon used by Alcoholics Anonymous, addicts generally have to get worse before they can get better.
When addicts talk about “chasing a high,” the metaphor can be extended to the losing battle of neurotransmitter levels.
[First published September 28, 2011]
Graphics Credit: http://www.keepcalm-o-matic.co.uk
Monday, April 22, 2013
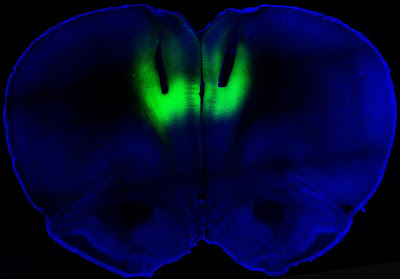
Let the Light Shine In: Addiction and Optogenetics
Study says laser light can turn cocaine addiction on and off in rats.
Francis Collins, the director of the National Institutes of Health (NIH), had one word for it: “Wow.”
Writing in the director’s blog at the online NIH site, Collins said that a team of researchers from NIH and UC San Francisco had succeeded in delivering “harmless pulses of laser light to the brains of cocaine-addicted rats, blocking their desire for the narcotic.”
Wow, indeed. It didn’t take long for the science fiction technology of optogenetics to make itself felt in addiction studies. The idea of using targeted laser light to strengthen or weaken signals along neural pathways has proven surprisingly robust. The study by the NIH and the University of California at San Francisco, published in Nature, showed that lab rats engineered to carry light-activated neurons in the prefrontal cortex could be deterred from seeking cocaine. Conversely, laser light used in a way that reduced signaling in this part of the brain led previously sober rats to develop a taste for the drug. As Collins described the work:
The researchers studied rats that were chronically addicted to cocaine. Their need for the drug was so strong that they would ignore electric shocks in order to get a hit. But when those same rats received the laser light pulses, the light activated the prelimbic cortex, causing electrical activity in that brain region to surge. Remarkably, the rat’s fear of the foot shock reappeared, and assisted in deterring cocaine seeking.
All this light zapping took place in a brain region known as the prelimbic cortex. In their paper, Billy T. Chen and coworkers said that they “targeted deep-layer pyramidal prelimbic cortex neurons because they project to brain structures implicated in drug-seeking behavior, including the nucleus accumbens, dorsal striatum and amygdala.” These three subcortical regions are rich in dopamine receptors. In rats that had been challenged with foot shocks before being offered cocaine, “optogenetic prelimbic cortex stimulation significantly prevented compulsive cocaine seeking, whereas optogenetic prelimbic cortex inhibition significantly increased compulsive cocaine seeking.”
What this demonstrates is that similar regions in the human prefrontal cortex, known to regulate such actions as decision-making and inhibitory response control, may be “compromised” in addicted people. This abnormally diminished excitability in turn “impairs inhibitory control over compulsive drug seeking…. We speculate that crossing a critical threshold of prelimbic cortex hypoactivity promotes compulsive behaviors”
This all sounds vaguely unsettling; sort of a cross between phrenology and lobotomy. But it is no such thing, and the study authors believe that stimulation of the prelimbic cortex “might be clinically efficacious against compulsive seeking, with few side effects on non-compulsive reward-related behaviors in addicts.” For now, the researchers confess that they don’t know whether the reduction in cocaine seeking is caused by altered emotional conditioning, or pure cognitive processing.
Actually, nobody expects optogenetics to be used in this way with humans. The thinking is that transcranial magnetic stimulation, the controversial technique that employs noninvasive electromagnetic stimulation at various points on the scalp to alter brain behavior, would be used in place of invasive zaps with lasers. Expect to hear about clinical trials to test this theory in the near future. David Shurtleff, acting deputy director at the National Institute on Drug Abuse (NIDA), said in a prepared statement that the research “advances our understanding of how the recruitment, activation and the interaction among brain circuits can either restrain or increase motivation to take drugs.”
Chen B.T., Yau H.J., Hatch C., Kusumoto-Yoshida I., Cho S.L., Hopf F.W. & Bonci A. (2013). Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking, Nature, 496 (7445) 359-362. DOI: 10.1038/nature12024
Photo credit: Billy Chen and Antonello Bonci
Wednesday, September 28, 2011
The Biology of Stimulants, or Why You Can’t Stay High Forever
An essay on the losing battle for perpetual reward.
The amphetamine high, like the cocaine high, is a marvel of biochemical efficiency. Stimulants work primarily by blocking the reuptake of dopamine molecules in the synaptic gap between nerve cells. Dopamine remains stalled in the gap, stimulating the receptors, resulting in higher dopamine concentrations and greater sensitivity to dopamine in general. Since dopamine is involved in moods and activities such as pleasure, alertness and movement, the primary results of using cocaine or speed—euphoria, a sense of well being, physical alertness, and increased energy—are easily understood. Even a layperson can tell when lab rats have been on a meth binge. The rapid movements, sniffing, and sudden rearing at minor stimuli are not that much different in principle from the outward signs of meth intoxication among higher primates.
Chemically, cocaine and amphetamine are very different compounds. Psychoactively, however, they are very much alike. Of all the addictive drugs, smoked cocaine and speed have the most direct and most devastatingly euphoric effect on the dopamine systems of the brain. Cocaine and amphetamine produce rapid classical conditioning in addicts, demonstrated by the intense cravings touched off by such stimuli as the sight of a building where the user used to buy or sell. Environmental impacts of this nature can produce marked blood flow increases to key limbic structures in abstinent addicts.
In clinical settings, cocaine users have a hard time distinguishing between equal doses of cocaine and Dexedrine, administered intravenously. As we know, it is the shape of the molecule that counts. The amphetamines are shaped like dopamine and norepinephrine, two of the three reward chemicals. Speed, then, is well suited to the task of artificially stimulating the limbic reward pathway. Molecules of amphetamine displace dopamine and norepinephrine in the storage vesicles, squeezing those two neurotransmitters into the synaptic gap and keeping them there. By mechanisms less well identified, cocaine accomplishes the same feat. Both drugs also interfere with the return of dopamine, norepinephrine, and serotonin molecules to their storage sacs, a procedure known as reuptake blocking. Cocaine works its effects primarily by blocking the reuptake of dopamine.
Amphetamine was once one of the most widely prescribed drugs in the pharmacological cornucopia. It exists in large part now as a recreational drug of choice, abuse, and addiction. The same is true of cocaine. It was replaced as a dental anesthetic long ago, in favor of non-addictive variants like Novocain. The same tragic list of statistical side effects that apply to abusers of alcohol, heroin and nicotine also apply to stimulant abusers: Increased risk of car accidents, homicides, heart attack, and strokes.
In the late 1990s, scientists at Johns Hopkins and NIDA showed that opiate receptors play a role in cocaine addiction as well. PET scans demonstrated that cocaine addicts showed increased binding activity at mu opiate receptors sites in the brain during active cocaine addiction. Take away the cocaine, and the brain must cope with too many empty dopamine and endorphin receptors. It is also easy to understand the typical symptoms of cocaine and amphetamine withdrawal: lethargy, depression, anger, and a heightened perception of pain. Both the cocaine high and the amphetamine high are easily augmented with cigarettes or heroin. These combinations result in “nucleus accumbens dopamine overflow,” a state of neurochemical super saturation similar to the results obtained with the notorious “speedball”—heroin plus cocaine.
With the arrival of smokable forms of cocaine and amphetamine, the race to pin down the biology of stimulation became even more urgent. Stimulants in smokable form—crack and ice—are even more rapidly addictive for addiction-prone users. “The reason has to do with the hydraulics of the blood supply,” a researcher at the University of Minnesota explained to me. “High concentrations are achieved with each inhalation, and sent right upstairs to the brain—but not all of the brain simultaneously. The target of the flow of blood is the limbic system, whereas the remainder of the brain is exposed to much milder concentrations.”
This extraordinarily concentrated jolt to the reward center is the reason why smokable cocaine and speed are able to pack such a wallop. The entire range of stimulative effects hits the ventral tegmental area and associated reward regions of the brain in seconds, and the focused nature of the impact yields an astonishingly pleasurable high.
But the long-term result is exactly the opposite. It may sound dour and religious, but the scientific fact of the matter is that continuous chemical pleasure extracts its fee in the end: The body’s natural stock of these neurotransmitters starts to fall as the brain, striving to compensate for the artificial flooding of the reward center, orders a general cutback in production. At the same time, the receptors for these neurotransmitters become excessively sensitive due to the frequent, often unremitting nature of the stimulation.
“It’s clear that cocaine causes depletion of dopamine, norepinephrine, serotonin—it is a general neurotransmitter depleter,” said my research source. “That may account for many of the effects we see after someone has stopped using cocaine. They’re tired, they’re lethargic, they sleep; they may be depressed, moody, and so on.” Continued abuse of stimulant drugs only makes the problem worse. One reason why cocaine and amphetamine addicts will continue to use, even in the face of rapidly diminishing returns, is simply to avoid the crushing onset of withdrawal. Even though the drugs may no longer be working as well as they once did, the alternative—the psychological cost of withdrawal—is even worse. In the jargon used by Alcoholics Anonymous, addicts generally have to get worse before they can get better.
When addicts talk about “chasing a high,” the metaphor can be extended to the losing battle of neurotransmitter levels.
Photo Credit: http://etinkata.blogspot.com
Monday, August 16, 2010
Chasing the Genes for Cocaine Addiction
Brain protein MeCP2 in the spotlight.
Dr. Edward Sellers, former director of the psychopharmacological research program at the University of Toronto’s Addiction Research Foundation once said to me: “Every cell, every hormone, every membrane in the body has got genetic underpinnings, and while many of the genetic underpinnings are similar in people, in fact there are also huge differences. So on one level, the fact that there is a genetic component to addiction is not very surprising. What is surprising is that you could ever have it show up in a dominant enough way to be something that might be useful in anticipating risk.”
If there existed a set of genes that predisposed people to alcoholism, and possibly other addictions, then these genes had to control the expression of something specific. That’s what genes did. However, back in the 1990s, addiction researchers could not even agree on the matter of where they should be looking for such physical evidence of genetic difference. In the brain? Among the digestive enzymes? Blood platelets? A gene, or a set of genes, coding for…what? What was it they were supposed to be looking for?
What set of genes coded for addiction?
Something about modern genetic research breeds a strong jolt of excitement. There is the promise of sudden discoveries, headlines, and great leaps forward toward cures for stubborn diseases. Even the most sober scientists seem to get enthused about gene hunting. The idea of curing a disease by locating a defective gene and repairing it is one of the brightest and fondest hopes in medicine. At least 3,000 medical disorders, including diabetes, cystic fibrosis, and some forms of Alzheimer’s are inherited diseases caused by defective genes passed on from generation to generation. But the premature announcements and retractions involving genes for everything from drinking to shyness has brought a hard-won maturity to the field.
These days, the hunt for evidence of genes influencing addiction is drilling very deeply into the molecular underpinnings of neural activity, in a wide-ranging effort to sort out the variety ofgene interactions involved in the genetic propensity for alcoholism and other addictions.
Researchers at Scripps discovered that cocaine increased levels of this regulatory protein in the brains of rats. So did fluoxetine , better known as Prozac, suggesting that the serotonergic system may be involved. “At that point,” according to lead author Paul Kenny, “we wanted to know if this increase was behaviorally significant—did it influence the motivation to take the drug?” Evidently it did. The higher the levels of MeCP2 in the brain, the higher the rats’ motivation to consume cocaine. When the researchers disrupted the expression of MeCP2 with a virus, the rats showed less interest in cocaine.
This is the first evidence that MeCP2 plays some as yet unexplained role in regulating vulnerability to cocaine addiction. Earlier this summer, investigators reported in Nature that another regulatory molecule known as MiRNA-212—a type of RNA involved in gene regulation--had the opposite effect, lessening the test animals’ interest in cocaine. The balancing act between MeCP2 and MiRNA-212 may help explain “the molecular mechanisms that control the transition from controlled to compulsive cocaine intake,” according to the paper, although the mechanisms that regulate this balance are not known.
One strong piece of evidence for this regulatory feedback loop was the finding that, while MeCP2 blocked miR-212 expression, the opposite was also true. “We still don’t know what exactly influences the activity levels of MeCP2 on miR-212 expression,” according to Kenny. “Now we plan to explore what drives it—whether it’s environmentally driven, and if genetic and epigenetic influences are important.” (For more on MeCP2, check this Lab Spaces post.)
NIDA director Nora Volkow said in an NIH press release that the work on MeCP2 “exposed an important effect of cocaine at the molecular level that could prove key to understanding compulsive drug taking.”
Graphics Credit: http://www.labspaces.net/
Im, H., Hollander, J., Bali, P., & Kenny, P. (2010). MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212 Nature Neuroscience DOI: 10.1038/nn.2615
Thursday, May 13, 2010
Cocaine Treatment and the Stroop Test
Treatment dropouts do poorly on color/word match.
It’s commonly used to demonstrate behavioral inhibition, but it’s also a nifty parlor game. It is called the Stroop Test, and it plays off the fact that people are far better at reading words than they are at intentionally ignoring them. To prove it, John Ridley Stroop’s 1935 Ph.D. thesis showed how difficult it is to interfere with the automatic processing of words. In the basic Stroop test, a list of color names is presented. However, the word green might be printed in red ink, and the word red might be printed in blue ink. The task is to quickly name not the word itself, but the color of the word. As an example, for the word “green” printed in red ink, the correct verbal answer is “red.” Because of a phenomenon called directed attention, this is hilariously difficult to do. The subject must actively inhibit the automatic response—reading the word—in order to do something else.
What’s all this got to do with drug addiction?
Psychologists have known for some time that drug craving focuses attention on drug-related stimuli in the environment, and draws attention away from environmental cues unrelated to drugs. Naturally, researchers began to wonder whether the Stroop test could be brought to bear on the matter of addiction, and employed as a tool with which to predict the likelihood of relapse among the addict population.
As researchers at the University of Wales have pointed out, “Decisions about drinking and drug use can be highly automatic, with users being unaware of the factors that influence their decisions.” At the same time, addicts are hyper-aware of addiction-related environmental stimuli, compared to non-addicts. As a result, “the automatic processing of addiction-related stimuli might elicit conditioned responses such as withdrawal… or they might invoke automatic patterns leading to substance use.”
In a recent study of treatment dropouts among 74 cocaine-addicted subjects,  published in Neuropsychopharmacology, Dr. Chris Streeter and coworkers at the Boston University School of Medicine and Harvard University provide strong evidence for the use of the Stroop Test as a diagnostic tool in addiction treatment. Variations on the Stroop Test were better predictors of dropout than addiction severity, depression, and other clinical variables. Dropouts took 24 per cent longer, on average, to finish the tests than cocaine addicts who stuck with treatment, the researchers reported. “These finding suggest that the Stroop test can be used to identify cocaine-dependent subjects at risk for treatment dropout,” say the researchers, and that it can serve as another instrument with which to “identify and tailor interventions of at risk individuals in the hope of improving treatment compliance.”
published in Neuropsychopharmacology, Dr. Chris Streeter and coworkers at the Boston University School of Medicine and Harvard University provide strong evidence for the use of the Stroop Test as a diagnostic tool in addiction treatment. Variations on the Stroop Test were better predictors of dropout than addiction severity, depression, and other clinical variables. Dropouts took 24 per cent longer, on average, to finish the tests than cocaine addicts who stuck with treatment, the researchers reported. “These finding suggest that the Stroop test can be used to identify cocaine-dependent subjects at risk for treatment dropout,” say the researchers, and that it can serve as another instrument with which to “identify and tailor interventions of at risk individuals in the hope of improving treatment compliance.”
Furthermore, other studies suggest that attentional bias may serve as a useful predictor of opiate relapse and smoking cessation failure as well.
Streeter, C., Terhune, D., Whitfield, T., Gruber, S., Sarid-Segal, O., Silveri, M., Tzilos, G., Afshar, M., Rouse, E., Tian, H., Renshaw, P., Ciraulo, D., & Yurgelun-Todd, D. (2007). Performance on the Stroop Predicts Treatment Compliance in Cocaine-Dependent Individuals Neuropsychopharmacology, 33 (4), 827-836 DOI: 10.1038/sj.npp.1301465
Photo Credit: http://www.edge.org
Sunday, March 14, 2010
The Cocaine Conundrum
Effective treatment remains elusive.
For addiction to cocaine, amphetamine, and other stimulants, the treatment picture has been complicated by the lack of any truly significant anti-craving medications. (See post, “No Pill for Stimulant Addiction"). The National Institute on Drug Abuse (NIDA) has yet to approve any medications for the treatment of either cocaine or amphetamine addiction.
Take the case of cocaine. Partly the problem stems from the direct effect cocaine has on dopamine transmission. The hunt for a pharmaceutical approach to blunt that effect is complicated by the problematic nature of dopamine receptors. Dopamine antagonist drugs like the antipsychotic drug haloperidol do not always block the stimulant rush. And their side effects, such as lethargy, emotional blunting, and tardive dyskinesia, make them unsuitable for ongoing addiction therapy. Conversely, some drugs that act as dopamine agonists turn out to be addictive in their own right. Many designer drugs are like that.
Because of all this, different approaches may be needed. The direct ride to the pleasure pathway provided by stimulants makes it difficult to tamper selectively with their effects. An antibody that would reduce cocaine consumption and sop up cocaine molecules in the brain, a kind of vaccine against cocaine, is one approach being pursued (See post, “Cocaine Vaccine Hits Snag”).
But other avenues of attack are being exploited. Scientists in NIDA’s Intramural Research Program are testing compounds that target certain proteins known as dopamine transporters. Transporters move dopamine molecules in and out of the synaptic gap between neurons in the brain. Interfering with that transportation system is another way of altering dopamine uptake, and it represents one active avenue of approach to the treatment of cocaine addiction.
The researchers tested Benztropine Mesylate (BZT), brand name Cogentin, one of a class of drugs known as anticholinergic suppressants commonly used in the management of Parkinson’s disease. What exactly does benztropine do? It possesses both anticholinergic (acetylcholine-blocking) and antihistaminic effects. It has chemical similarities to atropine, which is used for Parkinson’s and for heart disease.
To begin with, the researchers wanted to establish that benztropine itself is non-addictive. By substituting different BZT analogs for cocaine during self-administration testing on addicted rats, “two of the three BZT analogs that were tested significantly reduced drug self-administration… which indicates that those BZT analogs themselves have low potential for abuse.”
Next, the cocaine-addicted rats were given different BZT analogs before they got their cocaine. “When given before rats had access to cocaine in the self-administration chambers,” the researchers reported in the Journal of Pharmacology and Experimental Therapeutics, “two BZT analogs also significantly reduced the number of times the rats would press a lever to receive cocaine.” Monoamine uptake inhibitors were used as a control. The authors conclude that “these compounds are promising candidates for the development of medications for cocaine addiction.”
Hiranita, T., Soto, P., Newman, A., & Katz, J. (2009). Assessment of Reinforcing Effects of Benztropine Analogs and Their Effects on Cocaine Self-Administration in Rats: Comparisons with Monoamine Uptake Inhibitors Journal of Pharmacology and Experimental Therapeutics, 329 (2), 677-686 DOI: 10.1124/jpet.108.145813
Photo credit: http://www.drugabuse.gov
Sunday, March 7, 2010
The Perils of Fair-Weather Cocaine
The higher the temp, the higher the death rate.
As spring approaches, cocaine users might take note of further evidence of a connection between high ambient air temperatures and accidental overdoses.
A study published recently in the journal Addiction used mortality data from the Office of the Chief Medical Examiner in New York City from 1990 to 2006 to determine the frequency of cocaine-related overdoses (itself an enterprise fraught with uncertainty and argument over listed causes of death). The researchers cross-referenced the mortality data with temperature records from the National Oceanic and Atmospheric Association (NOAA).
As reported in Addiction Journal, “accidental overdose deaths that were wholly or partly attributable to cocaine use rose significantly as the weekly ambient temperature passed 24 degrees Celsius [75 degrees F].”
As reported in Addiction Journal, “accidental overdose deaths that were wholly or partly attributable to cocaine use rose significantly as the weekly ambient temperature passed 24 degrees Celsius [75 degrees F].”
Previous research, the authors write, had indicated that significantly higher temperatures—in the high 80s F--were required before cocaine mortality rates showed an increase. The researchers said they did not detect a corresponding rise in other types of drug overdoses during days over 75 degrees.
What is the mechanism connecting temperature to cocaine overdose? Cocaine intoxication raises core body temperature. Overheated cocaine users risk overdosing on smaller doses of the drug because their bodies are already under the strain of mild hyperthermia, or increased body temperature.
Specifically, the researchers from the University of Michigan and elsewhere found that above 75 degrees, there were 0.25 more drug overdoses per 1,000,000 residents per week for every two-degree rise in temperature, according to Addiction Journal. Applied to New York City, these numbers suggest and additional two cocaine deaths per week for every two degrees increase in average temperature over 75.
Lead author Dr. Amy Bohnert of the University of Michigan Medical School said in a press release that cocaine users are already “at a high risk of negative health outcomes and need public health attention, particularly when the weather is warm.” During the study period, New York City had average weekly temperatures in the >24 C range roughly seven weeks per year.
The idea is quite plausible, given that ambient air temperature can affect many other metabolic processes. Earlier investigations led to the discovery of a fairly well established diurnal AND seasonal variation for measurements of blood pressure. Researchers at Emory University data-mined 2 million electronic records of participating patients and discovered that the odds of having high blood pressure were lowest during the morning, and generally increased throughout the day. Seasonally, high blood pressure occurred more often in winter, and was at its lowest in the summer.
Graphics Credit: http://flatbushgardener.blogspot.com/
Bohnert, A., Prescott, M., Vlahov, D., Tardiff, K., & Galea, S. (2010). Ambient temperature and risk of death from accidental drug overdose in New York City, 1990-2006 Addiction DOI: 10.1111/j.1360-0443.2009.02887.x
Tuesday, January 19, 2010
Cocaine Vaccine Hits Snag
Some addicts risk OD to overcome its effects.
The National Institute on Drug Abuse (NIDA) has increasingly placed its bets on treating cocaine addiction with a vaccine rather than an anti-craving medication. And there is reason for this: No prominent candidates for anti-craving drug treatments have yet emerged from the research on cocaine and methamphetamine addiction.
However, there’s a catch: Some cocaine addicts appear willing to risk overdose in order to defeat a new cocaine vaccine, a recent study has shown.
The study, which appeared in the Archives of General Psychiatry, demonstrated that the TA-CD vaccine could blunt the effects of cocaine in some, but not all, patients. The vaccine works by causing the production of antibodies, which attach themselves to cocaine molecules, making the molecules too big too pass effectively through the blood-brain barrier.
Of 115 addicts involved in the study, only 38 % produced sufficient antibodies to dull the effects of cocaine, Rachel Saslow of the Washington Post reported. And among the high-antibodies group, only 53 % stayed free of cocaine 50 % of the time. “Immunization did not achieve complete abstinence from cocaine use,” said Thomas Kosten of Baylor college of Medicine, one of the authors of the paper.
Moreover, in some of the study participants for whom antibodies made cocaine a disappointing high, researchers found cocaine levels in the body to be as much as ten times higher than previous levels of usage—an obvious attempt to overcome the vaccine’s effectiveness. There were no overdoses, according to Kosten.
No researcher has claimed this as a complete breakthrough, in light of the fact that even those who responded well in the high-antibody group achieved a substantial reduction in cocaine use during the study period--but not abstinence. At this stage the work appears to be aimed more at dose reduction.
Despite the mixed results, NIDA director Nora Volkow characterized the work as “a promising step toward an effective medical treatment for cocaine addiction,” with the proviso that “larger follow-up studies confirm its safety and efficacy.” In an earlier interview with Addiction Inbox, Volkow also expressed excitement about another possible addiction vaccine: “Currently there are anti-nicotine vaccines in clinical testing, which are designed to capture the nicotine molecules while still in the bloodstream, thus blocking their entry in to the brain and inhibiting their behavioral effects. They appear to be effective in helping subjects who develop a high antibody response sustain abstinence over long periods of time. Even those people with a less robust antibody response to the vaccine, decreased their tobacco use. So this approach appears very promising.”
An earlier study by Margaret Haney and others at Columbian University Medical Center, published in Biological Psychiatry, had similar results: “The TA-CD vaccine substantially decreased smoked cocaine's intoxicating effects in those generating sufficient antibody.”
In both studies, roughly a quarter of participants made almost no antibodies at all in response to a vaccine injection.
A multi-site clinical trial of the vaccine, headed up by Kosten at Baylor, will begin sometime this spring.
Haney of Columbia told the Washington Post that people “have a mistaken view of how a vaccine might work, thinking of it as magic, where what it’s doing, at best, is blunting the effects. They get very excited, and it’s heartbreaking.” An earlier Addiction Inbox post on cocaine vaccination brought several emails from people asking where they could obtain the vaccine.
DrugMonkey at scienceblogs.com dissected the complicated study, particularly the different levels of antibodies generated in study participants, calling the vaccine “quite obviously not a silver bullet at present.” Furthermore: “Even for the high-responders the outcome was far from overwhelming, a 10 percentage improvement from 35% to 45% cocaine-free urines.”
Given how intractable to treatment addiction to stimulants has proven, any promising results at all are cause for cautious optimism. DrugMonkey writes: “We need new approaches and this immunopharmacotherapy stuff has potential.”
Monday, November 2, 2009
The Black Market for Seroquel

Speed freaks, coke heads, and antipsychotics.
Last week, writing on the Daily Beast web site, reporter Jeff Deeney profiled a startling underground market for the antipsychotic medication Seroquel (quetiapine). Deeney described street transactions in North Philadelphia for Quells or Suzie-Qs, as the drug is sometimes called. Seroquel, a drug developed for the treatment of schizophrenia and bipolar disorder, has developed an additional reputation as a “comedown” drug for stimulant abusers.
Seroquel, a so-called atypical antipsychotic, works by altering levels of dopamine. While some addicts have claimed that the drug is perfect for a cocaine or speed comedown, Seroquel has also been studied for its anti-craving properties when used for cocaine abstinence.
Why would a speed freak or a coke addict want to take a drug that might decrease their desire for their stimulant of choice? For the same reason that ecstasy users often take a morning-after dose of Prozac in a misguided attempt to compensate for possible damage to serotonin receptor arrays. Or because the drug is mildly sedating for some users. However, there may be more to it. Perhaps Seroquel is an effective anti-craving medication for cocaine and methamphetamine addicts, who misuse it as a drug to ease them through enforced periods of detox or lack of availability.
One high-traffic drug discussion site has shut down a long-standing thread on Seroquel with the warning: “Do not use Seroquel for a cocaine comedown.”
The fact that prescription Seroquel is available as a street drug, at least in some parts of the country, demonstrates the likelihood that physicians and psychiatrists are increasingly using it for off-prescription purposes—like drug detox. Deeney strongly suggests that this is the case: “Drug dealers, mandated to treatment as a condition of their probation or parole, are given off-label prescriptions for Seroquel, then sent right back to the street, where the pills can be sold for cash to users and other dealers.”
Increasing its appeal is Seroquel’s reputation for combining well with cocaine in a mixture known as a Q-Ball, or Rosemary’s Dolly—a variation on the heroin/cocaine mix known as a Speedball, to which Seroquel can also be added. An anonymous med student on a medical blog noted that “certain people say they love Seroquel when doing a speed-ball. Makes sense, think about it. It heightens the high of the heroin, it eases the crash of the cocaine.”
Seroquel’s ability to modulate the effect of illegal drugs means that the medication can possibly find a market both as a detox drug for stimulant abusers, and as an ingredient in the very stimulants they abuse.
By itself, Seroquel is not considered addictive. Some addicts told Deeney that the drug simply put them to sleep more quickly after a long meth run. Indeed, Seroquel is considered to be more sedating than similar antipsychotics such as Olanzapine and Aripiprazole. The larger issue, as the Daily Beast post makes clear, is that “Seroquel can have serious side effects including diabetes, a permanent Parkinson’s-like palsy called tardive dyskinesia, and sudden cardiac death.”
All of this confusing and sometimes contradictory input is coming well ahead of the clinical data, although a study in 2001, presented at the 4th International Conference on Bipolar disorder, found that quetiapine caused a significant reduction in cocaine use among a small group of cocaine-dependent subjects who also suffered from bipolar disorder. A report last year in the Journal of Clinical Psychopharmacology also showed positive results with cocaine users. Studies of quetiapine for the reduction of cocaine use are currently being undertaken by the Seattle Institute for Biomedical and Clinical Research.
Thursday, October 15, 2009
Another Round of Trials for Vigabatrin

Firm secures funding for anti-craving tests.
A Florida pharmaceutical company has secured financing for additional testing of the anti-addiction drug vigabatrin, despite the drug’s poor performance in earlier trials. Patrick J. McEnany, chairman and CEO of Catalyst Pharmaceuticals (CPRX) in Coral Gables, said the company would continue developing CPP-109 , its version of vigabatrin, for the treatment of cocaine and methamphetamine addiction.
Vigabatrin garnered early publicity on the basis of early trials suggesting it might be effective against stimulant addiction. Unlike alcohol and heroin, cocaine and speed have proven particularly resistant to treatment with other drugs designed to diminish craving. A drug that effectively reduced craving in abstinent cocaine and methamphetamine addicts would open up a potentially large and lucrative market.
Catalyst said it raised $3.97 million in a recent common stock offering from a group of investors including Federated Kaufmann Funds. Catalyst owns exclusive licensing rights to several patents related to vigabatrin from Brookhaven National Laboratory, reports Genetic Engineering and Biotechnology News. The company also owns patents or patent applications in more than 30 countries. Catalyst recently acquired worldwide rights to a related patent held by Northwestern University.
Earlier, the U.S. Food and Drug Administration (FDA) had given Fast Track designation to vigabatrin. The drug increases brain levels of GABA, an inhibitory transmitter. However, CPP-109 failed in a mid-stage treatment for cocaine addiction. Brian Bandell of the South Florida Business Journal reported that during the 12-week study, the drug did not help addicts stay cocaine-free, compared to a placebo group. In July, the company’s stock was trading at a 52-week low of 39 cents.
Last week, Catalyst said its decision to renew testing and development efforts with vigabatrin was due to a reanalysis of data from the earlier test. The company said the review showed that overall test subject compliance rates during the clinical trial may have been as low as 40 %. The company also said that early results with methamphetamine addiction were promising, but not statistically significant due to the small number of test subjects.
Last year, there was also a flurry of interest in vigabatrin as a weight loss drug. (See my earlier post). The FDA has yet to approve the drug for use in the U.S., citing concerns about reports of retinal damage in patients overseas. Catalyst said it had not uncovered any clinically significant visual abnormalities in its CPP-109 testing programs.
Vigabatrin, or gamma vinyl-GABA, is marketed in Europe as Sabril, and has existing clinical uses for the treatment of specific types of epilepsy and infant spasms.
Graphics Credit: www.dosewatch.com
Saturday, August 22, 2009
Who are Cocaine’s Primary Victims?

The answer may surprise you.
They are not necessarily the poor, the desperate, or the weak-willed. A National Institute of Drug Abuse (NIDA) study by Dr. Michael Nader and coworkers at Wake Forest University demonstrates that they are likely to be people with innately low levels of dopamine receptor availability. This flaw, possibly genetic, renders them more sensitive to the rewarding effects of cocaine. Put simply: Individuals with less dopamine naturally available in the brain may have an inherited predisposition for cocaine addiction. [Brains Scans at right: Dopamine receptor availability in yellow falls markedly after 6 and 12 months of cocaine self-administration.]
Dopamine D2 receptors, a crucial part of the brain’s primary reward system, are normally occupied by dopamine molecules—although at any given moment, many of the receptors are empty and remain available until a stimulus like cocaine increases dopamine levels and the empty receptors help mop up the excess. Dr. Nader believes that lower D2 receptor availability could be a precursor of addiction to drugs like cocaine. “Perhaps an individual with low availability gets a greater kick from cocaine because the drug-induced dopamine release stimulates a greater percentage of their receptors,” Dr. Nader told staff writer Lori Whitten in a recent edition of NIDA Notes. “Another possibility is that the drug prompts some individuals’ brain cells to release dopamine in particularly high quantities that are sufficient to fill the great majority of vacant D2 receptors, and this augments the high.”
An obvious question hangs over studies of this kind: Are the D2 receptor differences innate, or do they represent changes induced by drug use? To answer this question, Dr. Nader’s team worked with rhesus monkeys in order to take D2 density measurements with PET scans before the animals had ever been exposed to cocaine. Sure enough, the monkeys with the lowest baseline level of D2 receptor availability went on to self-administer cocaine at much higher rates than their D2-normal compatriots. Offering food to the low-dopamine animals did not prove to be a substitute of cocaine, so the effect does not appear to increase all kinds of reward.
There is no doubt that the use of cocaine itself does lead to a rapid reduction of available dopamine receptors, as the brain seeks to achieve a new equilibrium in the face of regular dosings of dopamine-active chemicals. In five monkeys that self-administered cocaine for a year, three of the monkeys showed a strong recovery of receptor availability after only a month of abstinence. However, two of the monkeys showed slower recovery of previous D2 receptor levels. Dr. Cora Lee Wetherington, a neuroscience researcher at NIDA, said that the research thus posed the question of whether people whose dopamine receptor levels recover more slowly during abstinence might prove to be those most likely to relapse.
Medications that increase D2 receptor availability without themselves being highly rewarding represent another promising avenue for treatment. The drugs most likely to help, Dr. Nader thinks, are drugs that act indirectly on dopamine levels through alterations of serotonin and GABA levels in the brain. In addition, researchers are pursuing environmental enrichment experiments in animals and human subjects. Some studies have shown that enriching the environment results in greater D2 receptor levels, Dr. Nader says.
Photo Credit: NIDA
Friday, May 1, 2009
Guest Post: Things Go Better with Meth

The Pepsi Challenge with controlled substances.
[Today’s post comes to us from Neurological Correlates, a blog devoted to the neuroscience of dysfunctional behavior. It was written by Swivelchair, who refers to himself as “an anonymous biopharma worker." It’s an excellent blog, one of the few that focuses on the biological basis of addiction.]
--------
Things go better with meth, as compared to cocaine, if you’re dopamine transporter challenged, anyway.
By Swivelchair
Methamphetamine is taken up more quickly, and lasts longer than cocaine. (Fowler et al, Abstract below).
And here’s something from Microgram Bulletin, October 2008, Published by the Drug Enforcement Administration Office of Forensic Sciences Washington, D.C. 20537: The DEA South Central Laboratory (Dallas, Texas) recently received a submission of approximately 4972 fake “kidney beans” (total net mass 3,210 grams), all containing a fine tan powder, suspected heroin. The “beans” were actually small plastic packets that had been painted to resemble kidney beans... Analysis of the powder... confirmed 90.3% heroin hydrochloride.
The perhaps undeniable point: probably the self-selecting population of people who are first drawn to drugs, and then become irretrievably addicted, are those who lack sufficient dopamine transport to feel fulfilled (or other insufficiency, depending on the choice of drug). They are, in essence, self-medicating, rather than using drugs for recreational use. I mean, you don’t load up kidney beans for recreational drug users.
I’m reminded of a friends’ younger brother, from a locally well-known family, whose arrest was reported as bringing in “the largest amount” of cocaine in those parts. His remark: He was a wholesaler, and the newspaper quoted street (”retail”) values, so the report inflated his inventory value. This was purely about money for him — he made far more money selling coke than any job he was qualified to do (which was, well, probably none, unless being a bon vivant and sparkling raconteur with insufficient money to fund a high rent party lifestyle qualifies as a profession, which it may). If the US were to decriminalize drug use, and fund a program to make an agonist which was not addictive (a la the whole methadone thing), probably we could solve much of the crime problem in the Western Hemisphere.
---------
“Fast uptake and long-lasting binding of methamphetamine in the human brain: comparison with cocaine.” Fowler JS, Volkow ND, Logan J, et. al. Medical Department, Brookhaven National Laboratory, Upton, NY 11973
Abstract from Neuroimage. 2008 Dec; 43(4):756-63.
“Methamphetamine is one of the most addictive and neurotoxic drugs of abuse. It produces large elevations in extracellular dopamine in the striatum through vesicular release and inhibition of the dopamine transporter. In the U.S. abuse prevalence varies by ethnicity with very low abuse among African Americans relative to Caucasians, differentiating it from cocaine where abuse rates are similar for the two groups. Here we report the first comparison of methamphetamine and cocaine pharmacokinetics in brain between Caucasians and African Americans along with the measurement of dopamine transporter availability in striatum.
Methamphetamine’s uptake in brain was fast (peak uptake at 9 min) with accumulation in cortical and subcortical brain regions and in white matter. Its clearance from brain was slow (except for white matter which did not clear over the 90 min) and there was no difference in pharmacokinetics between Caucasians and African Americans. In contrast cocaine’s brain uptake and clearance were both fast, distribution was predominantly in striatum and uptake was higher in African Americans. “Among individuals, those with the highest striatal (but not cerebellar) methamphetamine accumulation also had the highest dopamine transporter availability suggesting a relationship between METH exposure and DAT availability. Methamphetamine’s fast brain uptake is consistent with its highly reinforcing effects, its slow clearance with its long-lasting behavioral effects and its widespread distribution with its neurotoxic effects that affect not only striatal but also cortical and white matter regions. The absence of significant differences between Caucasians and African Americans suggests that variables other than methamphetamine pharmacokinetics and bioavailability account for the lower abuse prevalence in African Americans.”
Related Links
PET studies of d-methamphetamine pharmacokinetics in primates: comparison with l-methamphetamine and ( –)-cocaine. [J Nucl Med. 2007] PMID:17873134
Long-term methamphetamine administration in the vervet monkey models aspects of a human exposure: brain neurotoxicity and behavioral profiles. [Neuropsychopharmacology. 2008] PMID:17625500
Graphics Credit: methamphetaminetx.com
Wednesday, October 15, 2008
The Pharmacokinetics of Speed

Meth lingers longer than coke, targets different brain areas.
Scientists at the Brookhaven National Laboratory, already famous for their work on positron emission tomography (PET) scans, have traced the pathways by which methamphetamine lingers in the brain longer than cocaine. The Brookhaven Lab, managed by the U.S. Department of Energy (DOE) tested non-drug abusing volunteers. The results will be published in the November 1 issue of Neuroimage.
The researchers injected the 19 volunteers with radioactively tagged doses of the drugs. Scanning cameras then recorded the concentration and distribution of the tagged molecules. Both cocaine and methamphetamine enter the brain quickly—part of the reason why the two drugs are so reinforcing. However, cocaine clears the brain just as quickly, while meth does not. Moreover, the study demonstrated that methamphetamine is much more widely distributed throughout the brain than cocaine, which tends to exclusively target the dopamine-rich limbic reward pathways. “This slow clearance of methamphetamine from such widespread brain regions may help explain why the drug has such long-lasting behavioral and neurotoxic effects,” said Joanna Fowler, lead author of the study.
The researchers also looked at a more controversial hypothesis—widespread reports that methamphetamine abuse among African Americans is markedly lower than it is among Caucasians. These reports lead Fowler and her colleagues to question “whether biological or pharmacokinetic differences might explain this difference.”
The answer? Evidently not. According to a Brookhaven press release, “Surprisingly, the researchers found significant differences in cocaine pharmacokinetics between African Americans and Caucasians, with the African Americans exhibiting higher uptake of cocaine, a later rise to peak levels, and slower clearance.” When it came to speed, however, the scientists failed to detect any racial differences in uptake.
Fowler’s conclusion: “Variables other than pharmacokinetics and bioavailability account for the lower prevalence of methamphetamine abuse in African Americans.”
She added that “the differences observed for cocaine pharmacokinetics are surprising considering there are no differences in cocaine abuse prevalence between these two ethnic groups.”
This may come as a surprise to people who have been taught by news coverage and crime dramas to think of the crack problem as a “black problem.” But it may also indicate an inherent physiological preference for cocaine among African Americans, regardless of stated levels of abuse prevalence. As usual, more studies are needed.
Image Credit: Brookhaven National Laboratory News
Wednesday, July 16, 2008
Drugs for Cocaine Addiction

Researchers target GABA, noradrenaline.
According to Catalyst Pharmaceutical Partners, a company conducting research on drugs for the treatment of addiction, "The U.S. Food and Drug Administration has recognized that cocaine addiction is a 'serious, life-threatening condition for which there is no current drug treatment,' and the National Institute on Drug Abuse (NIDA) has stated that finding a pharmacological treatment for cocaine addiction is their number one research priority."
Other researchers view it differently, however. Allan Parry, a drug counsellor in Liverpool, U.K., told New Scientist that such work was "only likely to be relevant to a tiny minority of people. People often give up cocaine because their lifestyle changes or they just grow up."
Fighting fire with fire--using drugs to treat drug addiction--will likely remain a controversial approach for years to come.
What is the rationale for the use of drugs in the treatment of drug addiction? There are two basic approaches. Scientists look for medications that help patients initiate abstinence, and they look for drugs that help prevent relapse once the patient has achieved abstinence. The categories are not hard and fast. For example, a drug that effective reduces the reinforcing effects of cocaine by reducing the intensity of withdrawal can theoretically perform both functions at once. On the other hand, a drug that blunts the euphoric effects of cocaine--a drug that takes away the best of the buzz, no matter how much cocaine is ingested--can also succeed at the twin tasks of abstinence initiation and relapse prevention.
The search for medications with which to treat cocaine addiction has been in progress much longer than equivalent efforts aimed at methamphetamine addiction. One research target of long standing is modafinil, an odd-duck drug sold as Provigil for the treatment of narcolepsy. A mild stimulant, modafinil does a little bit of everything, pharmacologically tweaking dopamine, noradrenaline, anandamide and GABA receptor systems. Perhaps for this reason, the drug seemingly has been tried for almost everything, from Alzheimer's to atypical depression to jet lag. The U.S. military has reportedly shown some interest in it.
According to published research by Kyle M. Kampman in the June 2008 Addiction Science and Clinical Practice (PDF), modafinil-treated human subjects used less cocaine than placebo-using counterparts did in several recent small-scale studies. "In a double blind pilot trial with 62 cocaine-dependent patients, those who received modafinil submitted more cocaine-metabolite-free urine samples than placebo-treated patients (42 vs. 22 percent; Dackis et al., 2005)."
Propranolol, better known as the beta-blocker Inderal, works primarily by suppressing adrenaline and noradrenaline levels. In human studies to date, propranolol has shown itself most effective with the most severely cocaine-addicted patients. Studies by Kampman have shown that propanolol-treated patients stay in treatment longer than patients in control groups do.
Specific research on relapse prevention strategies has focused on GABA-enhancing drugs that inhibit cocaine reinforcement by secondarily blocking the dopamine surge characteristic of cocaine intoxication. In addition to vigabatrin, discussed in the previous post, topiramate is another particularly well-suited candidate for relapse prevention. Known as Topamax, and prescribed for seizures and migraines, the drug has shown early promise: "In a 13-week, double-blind, placebo-controlled pilot trial of topiramate involving 40 cocaine-dependent patients.... more of those on topiramate achieved at least 3 weeks of continuous abstinence (59 vs. 26 percent)."
Surprisingly, the granddaddy of all anti-addiction drugs--Antabuse--has made a comeback as a subject of study for cocaine addiction, even though it has never been spectacularly effective in its original application as a relapse prevention drug for alcoholics. Disulfiram, as it is known chemically, causes unpleasant physical sensations, including vomiting, when combined with even small amounts of alcohol. It does so by inhibiting the enzymes responsible for degrading alcohol. Even a little becomes too much. In similar fashion, disulfiram retards the breakdown of cocaine, leading to extremely high levels that induce paranoia and anxiety rather than a pleasurable, if extreme, high. At least four published trials have demonstrated reduced cocaine use in disulfiram-treated patients, according to Kampman's paper . One important downside to using Antabuse for cocaine addiction is that serious complications might occur if alcohol is added to the mix.
Finally, and still well into the future, is the prospect of relapse prevention therapy by means of a vaccine--an entirely different mechanism of approach. Research has shown that it is possible to produce "cocaine-specific antibodies that bind to cocaine molecules and prevent them from crossing the blood-brain barrier, thereby blunting the drug's euphoric and reinforcing effects," Kampman's paper asserts. A vaccine called TA-CD has tested well in preliminary studies.
Subscribe to:
Posts (Atom)